Chemistry Fun with Dr. Dave Cordes
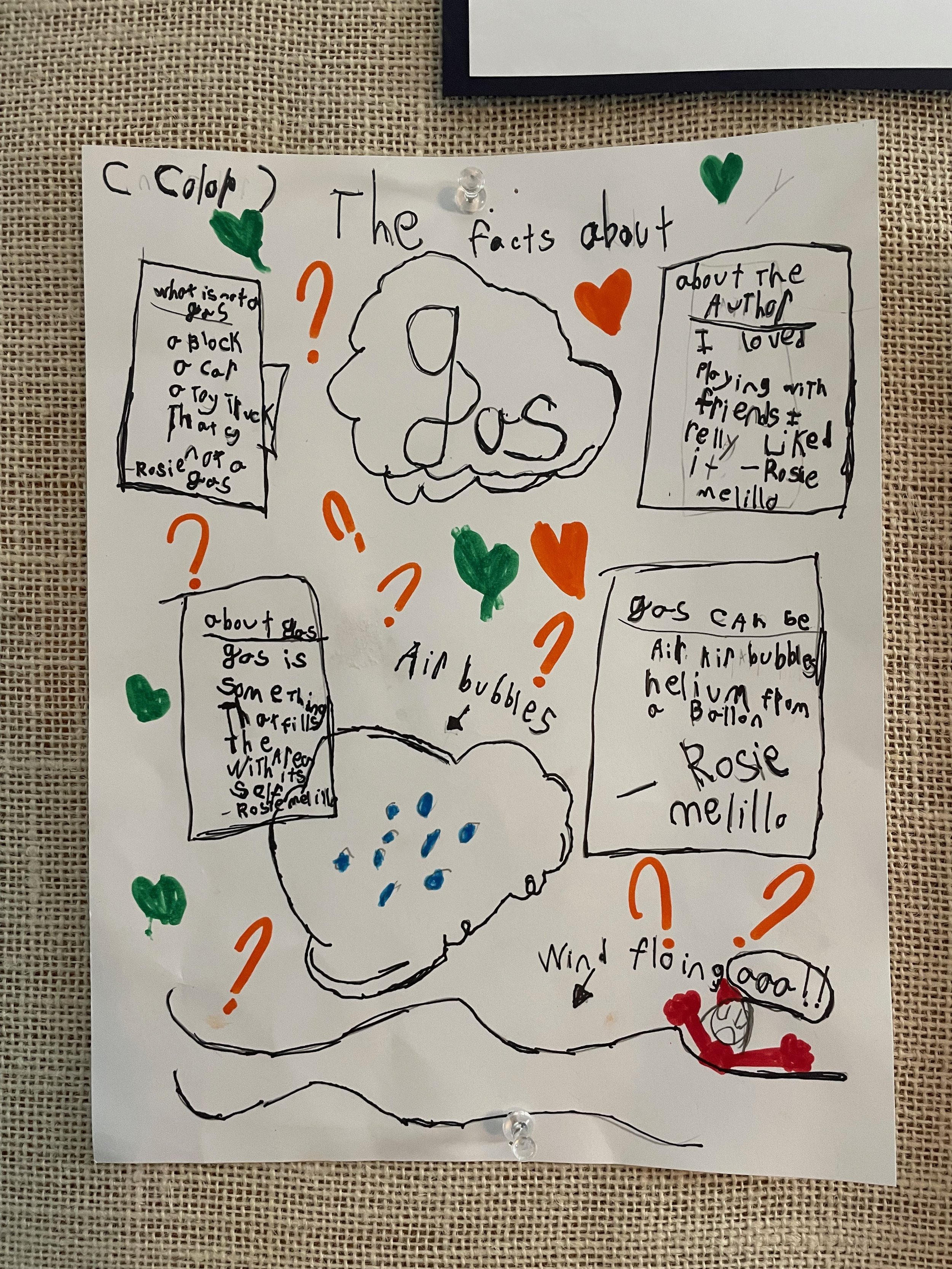
/ Aja AppelWhat a joy it is to be back together after our week apart! Over the break Mr. Mark was hard at work in our outdoor classroom. He cleaned up the meadow area and added a bamboo barrier to remind us not to trample on the growing plants. He also added a barrel filled with mud and clay for our potion-making endeavors, and completely drained and cleaned the pond so that it’s inviting for local wildlife. We are crossing our fingers that eventually frogs will decide to make their home here. Tony, the artist and engineer in charge of designing our new play structure, also made progress on the structure over break. We can’t wait for it be finished soon! This week we continued our exploration of chemistry. We did more science demonstrations to see what would happen when various solids- baking soda, alka seltzer tablets, and yeast mixed with different liquids such as vinegar and water. We discovered that chemical reactions can create gas molecules. In addition, students learned about the scientific method and worked individually or in pairs to design their own “dancing raisin” experiment. Students started with a question, gathered information, formed hypotheses, tested their hypotheses (being careful to only change ONE variable at a time), recorded data, and drew conclusions. Each group changed a different variable, such as types of sodas used, number of raisins used, jars with lids vs. no lids, amount of liquid used, size of jars, etc. We came up with some surprising results. The highlight of our week was a visit from associate chemistry professor Dr. David Cordes, who visited our classroom with a variety of science equipment. He showed us several examples of different types of solids, liquids, and gasses. We even got to see liquid metal and a solid form of carbon dioxide! Mr. Dave did multiple demonstrations that had us roaring with laughter and exclaiming in awe! It’s been immensely rewarding to witness the pure joy these students take in learning about science and to hear the depth of their scientific discussions. I never cease to be amazed by their questions, observations, and connections! A huge thank you to Dave for taking time out of his busy schedule to share his knowledge with us. In writer’s workshop students constructed persuasive pieces to convince others whether Nutella is a solid, liquid, or combination of both. Students will be sharing their writing pieces tomorrow afternoon. This week we also got to enjoy macarons from Paris (thank you, Wren!), worked on our class art project for the auction, helped the Whales write welcome letters to refugees, and received a fun Flat Stanley update from Montana. This morning we visited the Sea Lion classroom to check out the bridges they built during their recent bridge unit.